Generic Drugs and Medication Safety in November 2025: FDA Rules, Biosimilars, and Real-World Risks
When it comes to saving money on prescriptions, generic drugs, affordable versions of brand-name medications that meet the same safety and effectiveness standards as the original. Also known as non-brand drugs, they make up over 90% of prescriptions filled in the U.S. But not all generics are created equal—quality, manufacturing, and how they’re verified matter more than ever. In November 2025, the focus wasn’t just on cost—it was on trust. New data showed that some older generics made overseas, especially those with tiny profit margins, had higher rates of severe side effects. The FDA still approves them, but clinicians are asking tougher questions: Who made this? When was it inspected? And is it really the same as the brand?
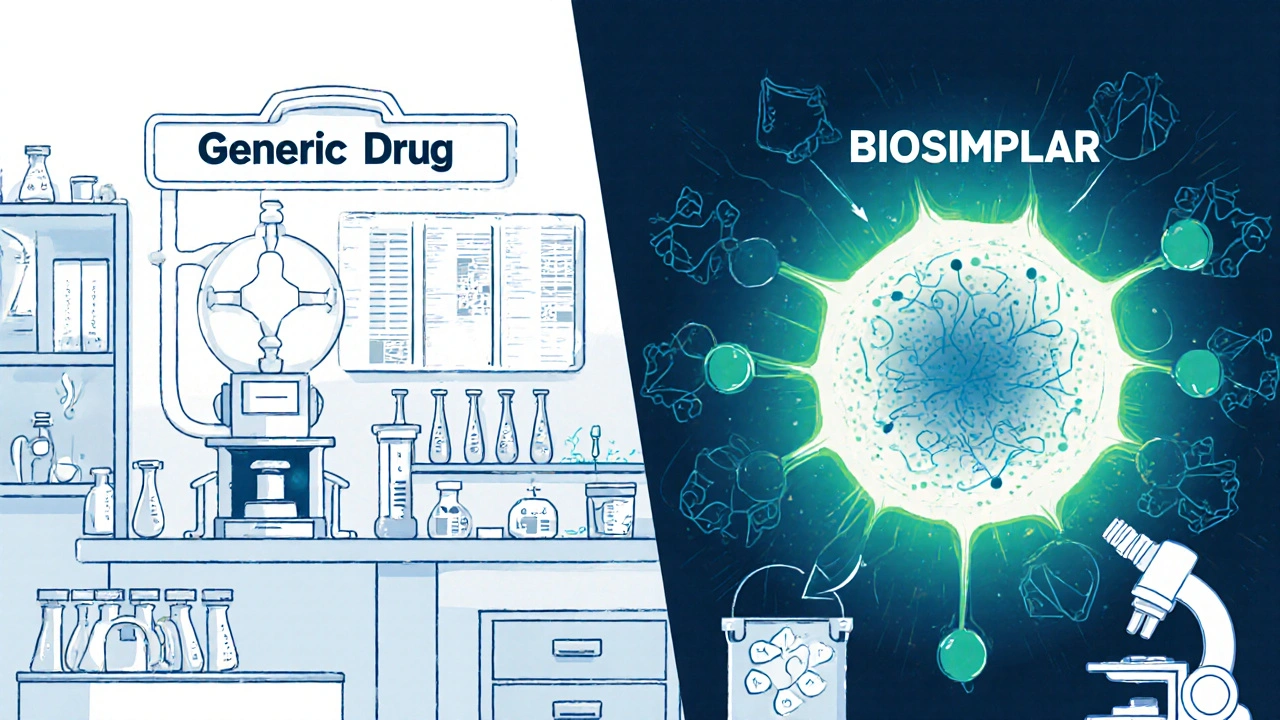
Biosimilars, complex, biologic drugs copied from highly advanced treatments like those for cancer or autoimmune diseases. Also known as biologic generics, they’re not simple chemical copies like traditional generics—they’re harder to replicate, require stricter testing, and cost more to produce. Unlike regular generics, biosimilars can’t be automatically swapped in at the pharmacy without a doctor’s OK. That’s because even tiny differences in manufacturing can change how the drug works in your body. Meanwhile, medication safety, the system of checks, clear labeling, and verification steps designed to prevent deadly errors in dosing, drug interactions, and misidentified medications. Also known as drug safety protocols, it’s the invisible shield between you and a hospital visit. Posts this month dug into real failures: look-alike drug names that cause mix-ups, trimethoprim raising potassium to dangerous levels, and PDE5 inhibitors crashing blood pressure when mixed with nitrates. These aren’t rare cases—they’re preventable.
What connects all these stories? Verification. Whether it’s confirming a dose change with SBAR protocols, checking for tall man lettering on labels, or understanding why a generic might cost 20x more than another, the theme is clear: assumptions kill. The FDA’s bioequivalence studies require generics to prove they behave the same in the body as the brand—but that’s just the starting point. Real-world use, patient age, kidney function, and other meds you take can change everything. That’s why a simple question like "Is this the right drug?" needs to be asked twice, not once.
November 2025 gave us a clearer picture: saving money on drugs doesn’t mean cutting corners. It means knowing what to ask, how to spot red flags, and understanding the science behind the pill in your hand. Below, you’ll find detailed guides on everything from antibiotic rashes and fluoroquinolone nerve damage to how calcium and vitamin D actually prevent fractures after 50. These aren’t theory pieces—they’re tools you can use today to protect yourself, your family, and your health.
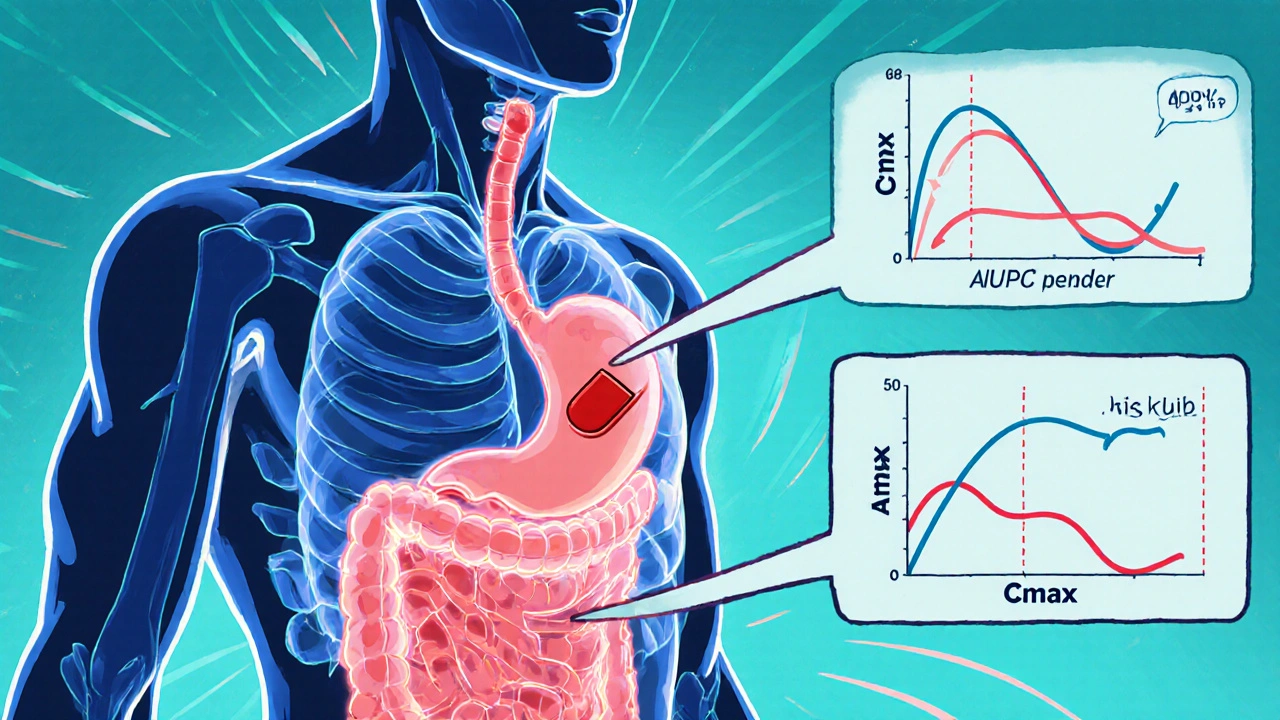
Bioequivalence Studies: What the FDA Requires Generic Drug Manufacturers to Prove
- Robin Tudge
- November 29, 2025
- 8 Comments
Learn what the FDA requires generic drug makers to prove through bioequivalence studies to ensure their products work just like brand-name drugs. Key criteria, exceptions, and recent updates explained.
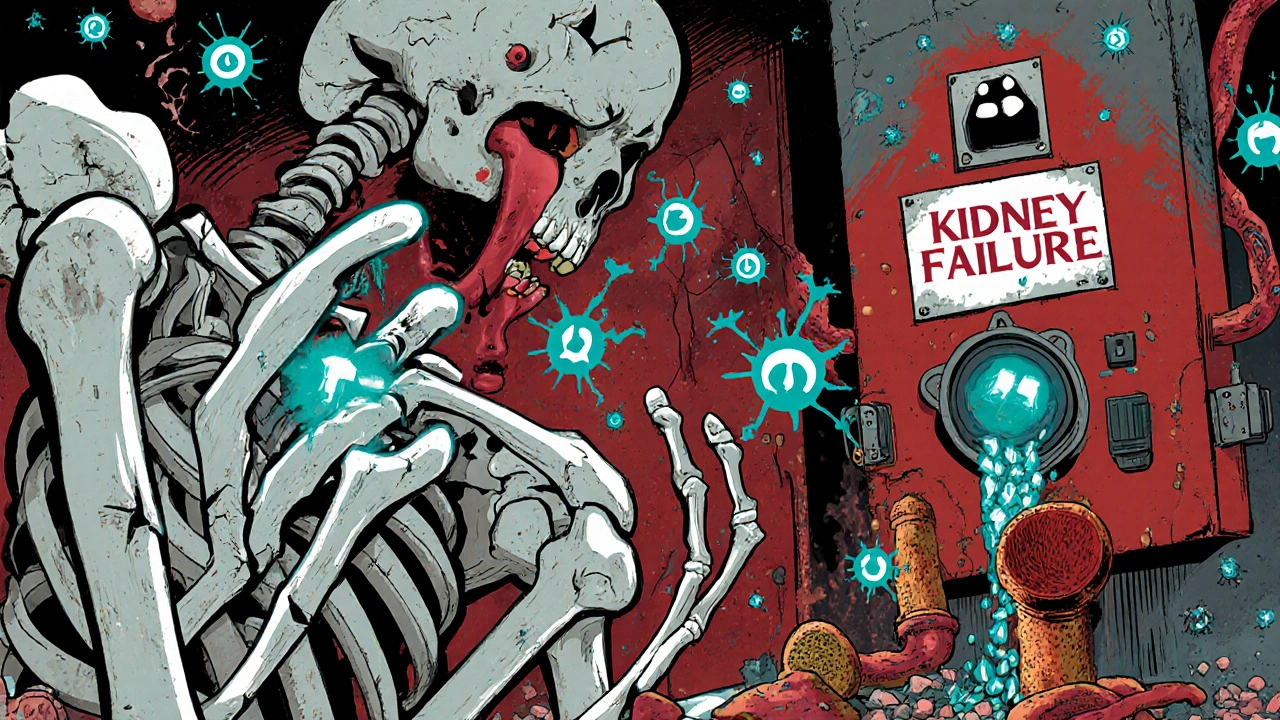
read moreMineral Bone Disorder in CKD: Understanding Calcium, PTH, and Vitamin D
- Robin Tudge
- November 28, 2025
- 14 Comments
CKD-MBD is a serious mineral disorder in kidney disease involving calcium, PTH, and vitamin D imbalances that lead to bone fractures and heart disease. Learn how it develops, how it's diagnosed, and what treatments actually work.
read moreHow to Identify Look-Alike Names on Prescription Labels
- Robin Tudge
- November 27, 2025
- 13 Comments
Learn how to spot dangerous look-alike drug names on prescription labels using tall man lettering, barcode scanning, and verification steps. Reduce medication errors with proven strategies used in U.S. hospitals.
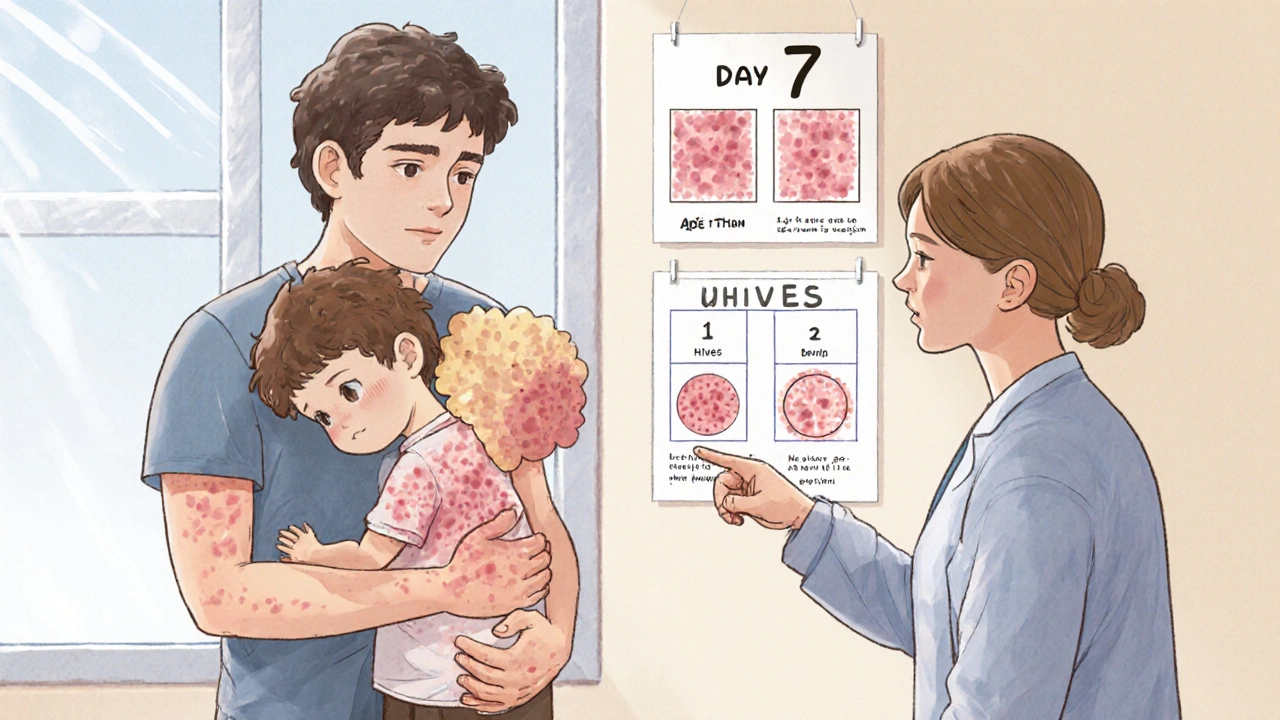
read moreAntibiotic Rashes: When to Stop the Drug and Call the Doctor
- Robin Tudge
- November 26, 2025
- 8 Comments
Most antibiotic rashes aren’t allergies - but knowing the difference can prevent dangerous mistakes. Learn when to stop the drug and when to keep going, based on rash type, timing, and symptoms.
read moreHow to Verify Dose Changes and Avoid Miscommunication in Healthcare
- Robin Tudge
- November 25, 2025
- 8 Comments
Learn how to verify medication dose changes safely using proven protocols, avoid deadly miscommunications, and use tools like SBAR and barcode scanning to prevent errors. Essential for nurses, pharmacists, and providers.
read moreBiosimilars vs Generics: Key Differences Explained
- Robin Tudge
- November 24, 2025
- 13 Comments
Biosimilars and generics both lower drug costs, but they’re not the same. Biosimilars are complex biologic copies with stricter rules, while generics are simple chemical copies. Understand the key differences in safety, cost, and substitution.
read moreFluoroquinolone Side Effects: Tendinopathy and Nerve Damage Risks
- Robin Tudge
- November 22, 2025
- 11 Comments
Fluoroquinolone antibiotics like ciprofloxacin and levofloxacin can cause permanent tendon rupture and nerve damage. Learn who’s at risk, how to recognize early signs, and safer alternatives for common infections.
read moreCost-Effectiveness Analysis: How Generic Drugs Save Money Without Sacrificing Care
- Robin Tudge
- November 21, 2025
- 15 Comments
Cost-effectiveness analysis reveals how generic drugs save billions by comparing prices and health outcomes. Learn why some generics cost 20x more than others-and how to spot real savings.
read moreContact Lens Safety: Hygiene, Solutions, and Wear Time
- Robin Tudge
- November 20, 2025
- 11 Comments
Learn the essential contact lens safety practices-hand hygiene, proper solutions, and wear time rules-to prevent eye infections and protect your vision. Follow CDC and AOA guidelines to avoid preventable vision loss.
read morePDE5 Inhibitors and Nitrates: How They Cause Dangerous Blood Pressure Drops
- Robin Tudge
- November 19, 2025
- 11 Comments
PDE5 inhibitors like Viagra and Cialis can cause life-threatening drops in blood pressure when taken with nitrates. Learn how this interaction works, how long to wait between doses, and what to do if it happens.
read more