When you hear "generic drug," you probably think of a cheaper version of your prescription pill-same active ingredient, same effect, lower price. But what about biosimilars? They’re also cheaper alternatives to brand-name medicines, yet they’re not the same as generics. Confusing? You’re not alone. Many patients and even some doctors mix them up. The truth is, biosimilars and generics are built on completely different science, regulated under different rules, and behave differently in your body.
What Are Generics, Really?
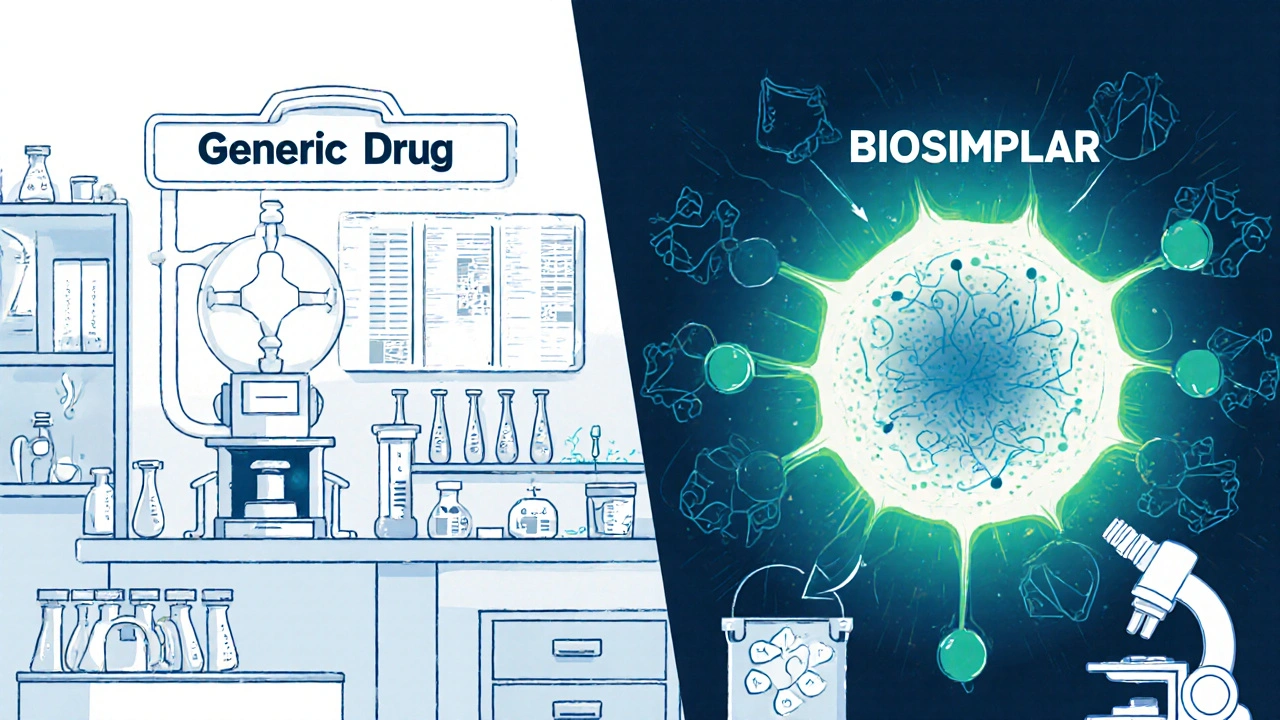
Generics are copies of small-molecule drugs. These are chemicals made in a lab, with a simple, well-defined structure. Think ibuprofen, metformin, or lisinopril. Once the patent on the original drug expires, other companies can make exact copies. They don’t need to run new clinical trials because they prove they work the same way in your body-same absorption, same breakdown, same results. The FDA requires them to be bioequivalent, meaning they deliver the same amount of medicine into your bloodstream as the brand-name version, within a very tight range.That’s why generics cost 40% to 50% less. The manufacturing process is straightforward. A chemical formula is replicated. No living cells involved. No complex purification. Just chemistry. As of 2022, over 10,000 generic drugs were approved in the U.S., and they make up about 90% of all prescriptions filled. Yet they account for only 20% of total drug spending because they’re so affordable.
What Are Biosimilars?
Biosimilars are copies of biologic drugs. These aren’t simple chemicals. They’re large, complex proteins made from living cells-like yeast, bacteria, or animal cells. Examples include Humira (adalimumab), Enbrel (etanercept), and Herceptin (trastuzumab). These drugs treat serious conditions like rheumatoid arthritis, Crohn’s disease, and cancer.Because they come from living systems, no two batches are exactly alike. Even the original manufacturer can’t make them perfectly identical every time. That’s why a biosimilar isn’t a copy-it’s a highly similar version. The FDA says it must show no clinically meaningful differences in safety, purity, or potency compared to the original. But proving that takes a lot more than just testing blood levels.
Manufacturers of biosimilars don’t have access to the original company’s secret production process. They have to reverse-engineer it. That means developing their own cell lines, their own purification methods, their own formulas. Then they run hundreds of tests-analyzing structure, function, stability, and how the body responds. Animal studies. Pharmacokinetic studies. Sometimes small clinical trials. All to prove similarity.
Why Biosimilars Cost Less-But Not As Much
Biosimilars save money, but not as dramatically as generics. You might expect a 50% discount, but the reality is closer to 15% to 33%. Why? Because developing a biosimilar costs $100 million to $200 million. That’s 20 to 50 times more than making a generic drug, which typically runs $2 million to $5 million.The complexity doesn’t stop at development. Manufacturing biosimilars requires sterile facilities, specialized equipment, and strict environmental controls. A single batch of a monoclonal antibody biosimilar can weigh 148 kilodaltons-over 700,000 times heavier than a molecule like aspirin. That’s not something you can produce in a standard pharmacy lab.
And because biosimilars are so complex, the FDA requires more data. More testing. More time. All of that adds up to higher costs, which get passed on to consumers-even if they’re still cheaper than the original biologic.
Substitution: Can Your Pharmacist Switch Them?
This is where things get really different. With generics, your pharmacist can swap the brand-name drug for the generic without asking your doctor. That’s called automatic substitution, and it’s legal in all 50 states.With biosimilars? Not so fast. Only biosimilars that are labeled as interchangeable can be substituted without a doctor’s approval. And as of November 2023, only 7 out of the 42 FDA-approved biosimilars have that status. The rest? Your doctor has to specifically write "dispense as written" or you’ll get the original brand.
Why the restriction? Because biologics can trigger immune reactions. Even tiny differences in structure might cause your body to react-leading to reduced effectiveness or unwanted side effects. For someone stabilized on Humira, switching to a non-interchangeable biosimilar without monitoring could be risky. That’s why the American Society of Health-System Pharmacists and the American College of Rheumatology urge caution.
Regulatory Pathways: Two Different Systems
Generics are approved under the Hatch-Waxman Act of 1984. The process is streamlined: prove bioequivalence, and you’re in.Biosimilars? They follow the Biologics Price Competition and Innovation Act (BPCIA) of 2009. It’s a step-by-step process that starts with analytical testing, moves to animal studies, then clinical trials if needed. The FDA doesn’t just check if the drug works-it checks if it’s structurally and functionally the same at a molecular level.
And the data exclusivity periods are different. Brand-name biologics get 12 years of market protection before biosimilars can even apply. Brand-name small-molecule drugs? Only 5 years. That’s a huge gap in how long companies can charge high prices before competition kicks in.
Market Reality: Generics Dominate, Biosimilars Are Rising
Generics are everywhere. They’re in your local pharmacy, your Medicare Part D plan, your hospital formulary. They’re the default choice for most prescriptions.Biosimilars? They’re still a small slice of the pie. Less than 3% of the biologics market in the U.S. as of 2023. But that’s changing fast. Major biologics like Humira, Enbrel, and Rituxan are losing patent protection. The first interchangeable biosimilar for Humira hit the market in January 2024 with a 35% discount. More are coming.
Compare that to the global market: biosimilars are growing at 23.8% per year. In Europe, they make up 35% of the biologics market. The U.S. is playing catch-up, but the Inflation Reduction Act of 2022 is pushing for lower out-of-pocket costs for biologics, which will help biosimilars gain ground.
What This Means for You
If you’re taking a small-molecule drug-like a blood pressure pill or an antidepressant-you’re likely already using a generic. No confusion. No questions. Just savings.If you’re on a biologic-say, for psoriasis, Crohn’s, or cancer-you might soon be offered a biosimilar. Ask your doctor: Is it interchangeable? Has it been used in patients like me? What are the risks if I switch?
Don’t assume a biosimilar is just a "generic biologic." It’s not. It’s a more complex, more expensive, more carefully studied alternative. But it’s still a valid option that can lower your costs without sacrificing safety.
For patients, the key is communication. Know what you’re taking. Ask about alternatives. Understand whether substitution is allowed. And don’t be afraid to ask your pharmacist or doctor to explain the difference.
Common Myths Debunked
- Myth: Biosimilars are just generics for biologics. Truth: They’re fundamentally different in science, regulation, and safety considerations.
- Myth: Switching from a biologic to a biosimilar is dangerous. Truth: For interchangeable biosimilars, studies show no increased risk. For non-interchangeable, it’s done cautiously with monitoring.
- Myth: Biosimilars are less effective. Truth: They’re proven to work just as well as the original in clinical trials.
- Myth: All biosimilars are the same. Truth: Each one is developed independently. One biosimilar for Humira may behave differently than another.
Where to Find Reliable Info
The FDA’s Purple Book lists all approved biological products, including biosimilars and interchangeable products. It’s not as user-friendly as the Orange Book (which lists generics), but it’s the official source. The Biosimilars Forum and the American College of Rheumatology also offer patient-friendly guides.For pricing, check GoodRx or NeedyMeds. Many biosimilars now have coupons or patient assistance programs, especially as more competitors enter the market.
Are biosimilars safe?
Yes. The FDA requires biosimilars to show no clinically meaningful differences in safety, purity, or potency compared to the original biologic. Thousands of patients have used them without increased risk of side effects. However, because they’re made from living cells, they carry a small risk of immune reactions-this is why switching must be monitored, especially for patients with autoimmune conditions.
Can I be switched from my biologic to a biosimilar without my doctor’s permission?
Only if the biosimilar has been designated as "interchangeable" by the FDA-and even then, state laws vary. As of 2023, only 7 out of 42 approved biosimilars have that status. For all others, your doctor must specifically prescribe the biosimilar. Pharmacies cannot automatically substitute them like they do with generics.
Why are biosimilars more expensive to make than generics?
Biosimilars are made from living cells, not chemicals. This requires complex manufacturing processes, strict environmental controls, and hundreds of analytical tests to prove similarity. The original manufacturer’s process is a trade secret, so biosimilar makers have to reverse-engineer it from scratch. Generics, by contrast, are simple chemical copies with well-known formulas.
Do biosimilars work as well as the original biologic?
Yes. Clinical trials show that biosimilars produce the same results as the original drug in terms of effectiveness, safety, and side effects. The FDA requires this before approval. For example, biosimilars for Humira have been shown to control arthritis symptoms just as well as the brand-name version in multiple studies.
What’s the difference between biosimilars and biologics?
Biologics are the original, brand-name drugs made from living organisms. Biosimilars are follow-on versions that are highly similar but not identical. Think of it like two identical-looking cars built by different factories-they look the same and drive the same, but their parts were made in slightly different ways. The original is the brand-name biologic; the biosimilar is the copy.
Will my insurance cover a biosimilar?
Most insurance plans, including Medicare Part D, now prefer biosimilars over brand-name biologics because they cost less. Some require you to try a biosimilar first before covering the original. Check your plan’s formulary or ask your pharmacist. The Inflation Reduction Act also helps lower your out-of-pocket costs for biologics and their biosimilars.
What’s Next?
The future of biosimilars looks promising. More patents are expiring. More companies are entering the market. And regulators are working to simplify the path to approval and interchangeability. By 2028, analysts predict biosimilars could capture 25% to 30% of the U.S. biologics market-up from under 3% today.For patients, that means more choices. Lower costs. Better access to life-changing treatments. But it also means staying informed. Know what you’re taking. Ask questions. Don’t let confusion stop you from saving money-or from getting the care you need.

All Comments
Karen Willie November 25, 2025
Just wanted to say this was one of the clearest explanations I’ve read on biosimilars vs generics. As someone managing a chronic condition, I’ve been nervous about switching, but now I feel more informed. Thanks for breaking it down without jargon.
Patricia McElhinney November 27, 2025
Actually, the FDA’s definition of ‘no clinically meaningful differences’ is misleading-there are always differences, and the data is often cherry-picked. The studies are underpowered, and post-market surveillance is laughably inadequate. You think you’re saving money, but you’re just gambling with your immune system.
Rachel Villegas November 27, 2025
It’s fascinating how the regulatory frameworks reflect the underlying science. Hatch-Waxman was designed for small molecules, and BPCIA had to evolve to handle biological complexity. The 12-year exclusivity period isn’t just corporate greed-it’s recognition that biologics require a decade of R&D just to get to the starting line.
Agastya Shukla November 28, 2025
From a pharmacokinetic standpoint, the structural heterogeneity of biosimilars introduces stochastic variability in Fc receptor binding, which can modulate ADCC and CDC activity-factors not captured in standard bioequivalence assays. That’s why interchangeability requires immunogenicity profiling across multiple cohorts, not just Cmax and AUC metrics.
Josh Zubkoff November 28, 2025
Okay so let me get this straight-pharma companies spend $200 million to make a copy of a drug that’s already been proven safe, and then they charge $30,000 a year for it, and we’re supposed to be thrilled because it’s ‘only’ 30% cheaper? Meanwhile, my deductible is $8,000 and my insulin costs $400 a vial. This isn’t innovation, it’s a shell game. The real biosimilar is the one in the boardroom, sipping champagne while the rest of us ration pills.
fiona collins November 29, 2025
Clear. Accurate. Needed.
Shivam Goel November 30, 2025
And yet… the FDA approved 7 interchangeable biosimilars… but only 3 have actually been dispensed as such… why? Because pharmacists are terrified of liability… and doctors don’t know the difference… and patients are scared… and the system is broken…
Amy Hutchinson December 2, 2025
Wait so you’re telling me I can’t just get the cheap version like with my blood pressure meds? My insurance said ‘biosimilar’ but my doctor said ‘nope’… so what am I supposed to do? Fight my insurer? Fight my doctor? I’m just trying to not go broke.
Archana Jha December 2, 2025
They say biosimilars are safe but have you heard about the 2019 German study where 12 patients developed anti-drug antibodies after switching? They buried it. Big Pharma owns the FDA. The whole system is rigged. They want you dependent. They don’t want you healthy. They want you on the same drug for life. Biosimilars? Just a trap to make you think you’re getting a deal while they keep you hooked.
Aki Jones December 4, 2025
Let’s be real: the ‘interchangeable’ label is a marketing ploy. The clinical trials are funded by the same companies that make the original biologics. The data is curated. The adverse events are underreported. And now they’re pushing this under the Inflation Reduction Act like it’s some kind of victory? It’s a Trojan horse. You think you’re saving money, but you’re just being guinea pigs for corporate R&D.
Andrew McAfee December 5, 2025
In India we’ve had biosimilars for over a decade-Humira copies cost $1500 a year instead of $7000. People live longer. Kids get to go to school. The science is solid. The fear? That’s just Western privilege talking. We didn’t wait for permission to save lives.
Arup Kuri December 5, 2025
People are so naive. They think cheaper means better. But this is the same system that gave us Vioxx and fen-phen. Biosimilars are the new opioid. A quiet crisis. You think your rheumatologist has your best interests at heart? They get kickbacks from the manufacturers. The FDA is asleep at the wheel. Wake up.
Elise Lakey December 6, 2025
I’ve been on a biologic for 6 years. My doctor offered a biosimilar last year. I asked for the data. She showed me the studies. I read them. I felt safe. I switched. No issues. It’s not magic. It’s science. And it’s working. Thank you for writing this-people need to hear this without fearmongering.