Tag: FDA generic drugs
Bioequivalence Explained: FDA Requirements to Prove Generic Drug Equivalence
- Robin Tudge
- January 8, 2026
- 8 Comments
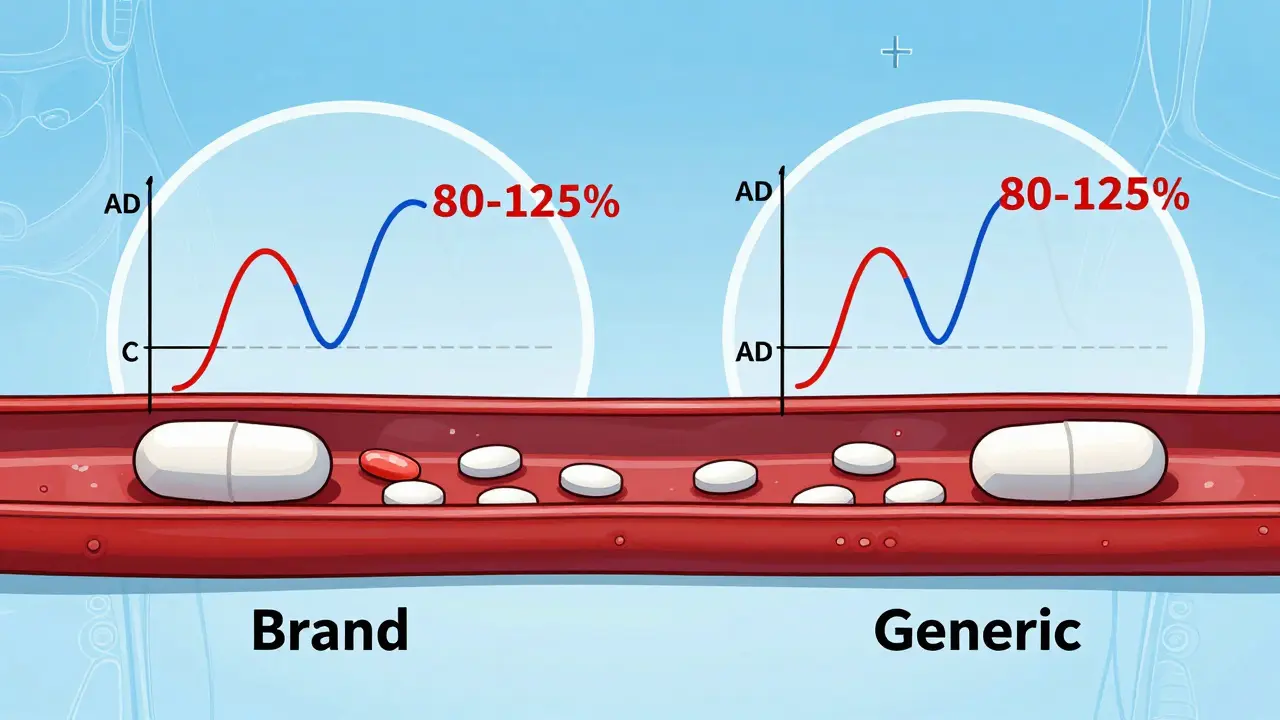
Learn how the FDA ensures generic drugs work just like brand-name ones through bioequivalence testing. Understand the 80-125% rule, what it really means, and why generics are safe and effective.
read morePost-Market Studies on Generic Drug Safety: What Happens After Approval
- Robin Tudge
- December 27, 2025
- 14 Comments
Generic drugs are safe for most people, but post-market studies reveal hidden safety issues that only appear after widespread use. Learn how the FDA tracks problems, why manufacturer differences matter, and what you can do to stay protected.
read moreBioequivalent Medications: What the Term Really Means
- Robin Tudge
- December 12, 2025
- 14 Comments
Bioequivalent medications are generic drugs proven to work the same as brand-name versions in the body. Learn how the FDA tests them, why they're safe, and when to be cautious.
read more