ANDA Approval: What It Means for Generic Drugs and Your Wallet
When you pick up a generic pill at the pharmacy, chances are it got through something called ANDA approval, a streamlined process by the U.S. Food and Drug Administration that lets generic versions of brand-name drugs enter the market. Also known as Abbreviated New Drug Application, it’s the backbone of affordable medicine in the U.S. This isn’t a shortcut—it’s a smart system. The FDA doesn’t require generic makers to repeat every clinical trial the original drug went through. Instead, they prove their version is the same in active ingredient, strength, dosage form, and how it works in your body. If it passes, it’s approved to sell at a fraction of the cost.
That’s why generic drugs, chemically identical copies of brand-name medications approved under the ANDA process make up over 90% of prescriptions filled in America. But not all generics are created equal. Some come from factories with strict quality controls, others from overseas plants with a history of FDA warnings. That’s why FDA inspections, regular checks on manufacturing sites to ensure safety and consistency matter so much. A 2023 study found that older, low-margin generics made in certain regions had higher reports of side effects—mostly because of inconsistent manufacturing, not the drug itself. The ANDA approval doesn’t guarantee perfection, but it does mean the drug meets baseline safety and effectiveness standards.
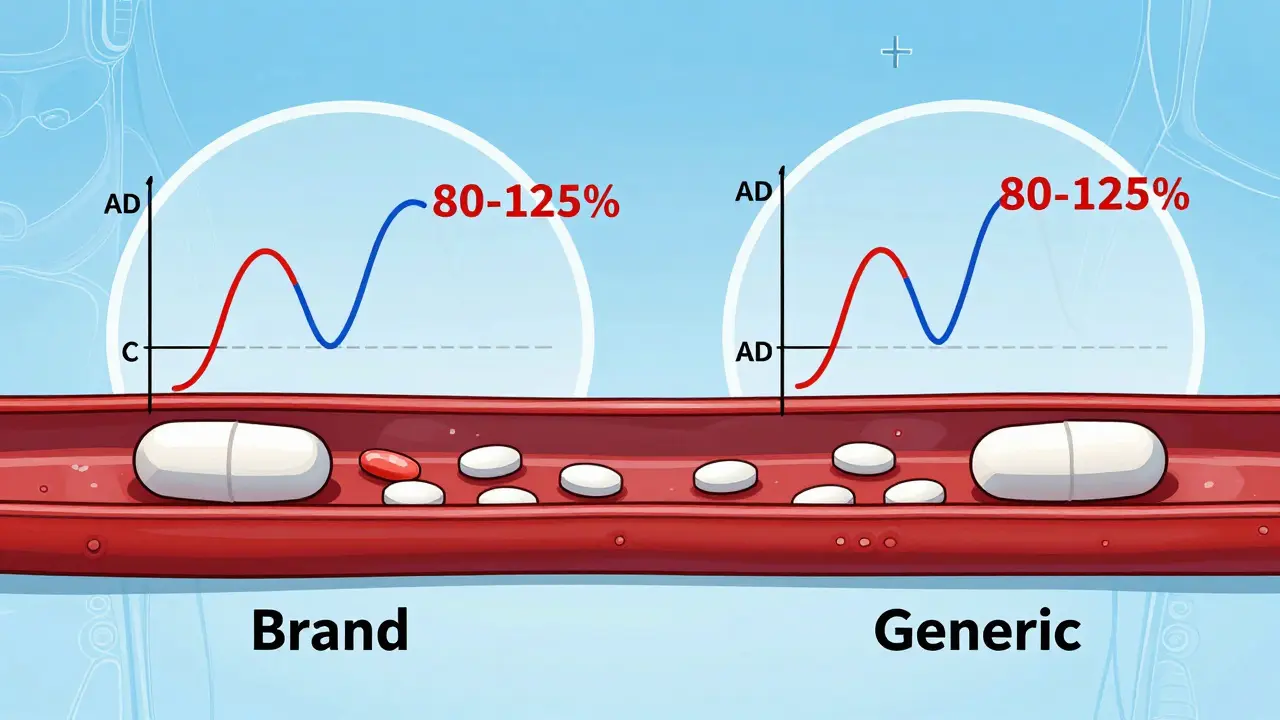
Behind every approved generic is a legal battle, a patent clock, and a financial incentive. When a brand-name drug’s patent expires, companies rush to file an ANDA. Some even launch authorized generics, the exact same drug as the brand, sold under a different label at lower prices—often by the brand company itself—to keep market share. This isn’t fraud. It’s capitalism. And it’s why you might see two identical pills on the shelf, one labeled "Brand" and one labeled "Generic," with the generic costing 20 times less.
You don’t need to be a pharmacist to understand this. If you’re on a chronic medication—thyroid pills, blood pressure drugs, antidepressants—you’re likely taking something that went through ANDA approval. The system works when it’s transparent. When inspections are frequent. When manufacturers can’t cut corners without consequences. The posts below dive into real cases: how quality issues slip through, why some generics cost more than others, and how patients and clinicians are starting to question what "equivalent" really means. What you’ll find here isn’t theory. It’s what’s happening in clinics, pharmacies, and living rooms across the country.
Bioequivalence Explained: FDA Requirements to Prove Generic Drug Equivalence
- Robin Tudge
- January 8, 2026
- 8 Comments
Learn how the FDA ensures generic drugs work just like brand-name ones through bioequivalence testing. Understand the 80-125% rule, what it really means, and why generics are safe and effective.
read moreBioequivalence Studies: What the FDA Requires Generic Drug Manufacturers to Prove
- Robin Tudge
- November 29, 2025
- 8 Comments
Learn what the FDA requires generic drug makers to prove through bioequivalence studies to ensure their products work just like brand-name drugs. Key criteria, exceptions, and recent updates explained.
read more