Tag: bioequivalence standards
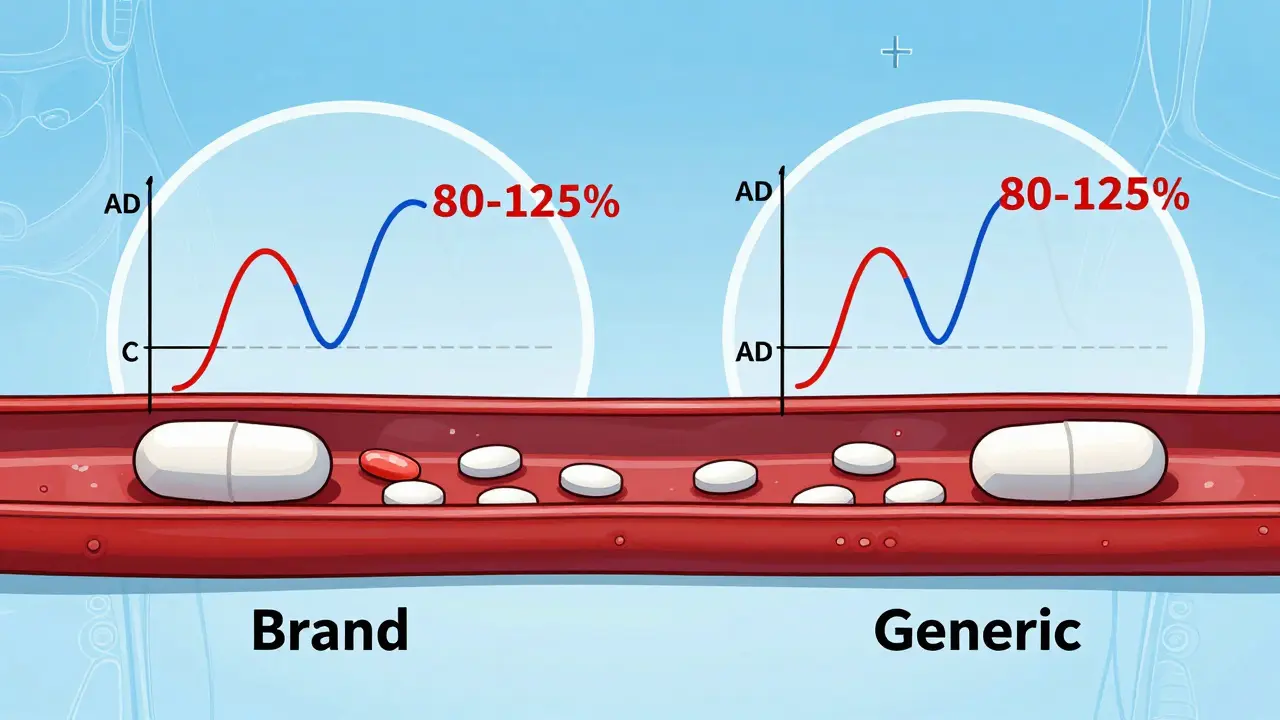
Bioequivalence Explained: FDA Requirements to Prove Generic Drug Equivalence
- Robin Tudge
- January 8, 2026
- 1 Comments
Learn how the FDA ensures generic drugs work just like brand-name ones through bioequivalence testing. Understand the 80-125% rule, what it really means, and why generics are safe and effective.
read more