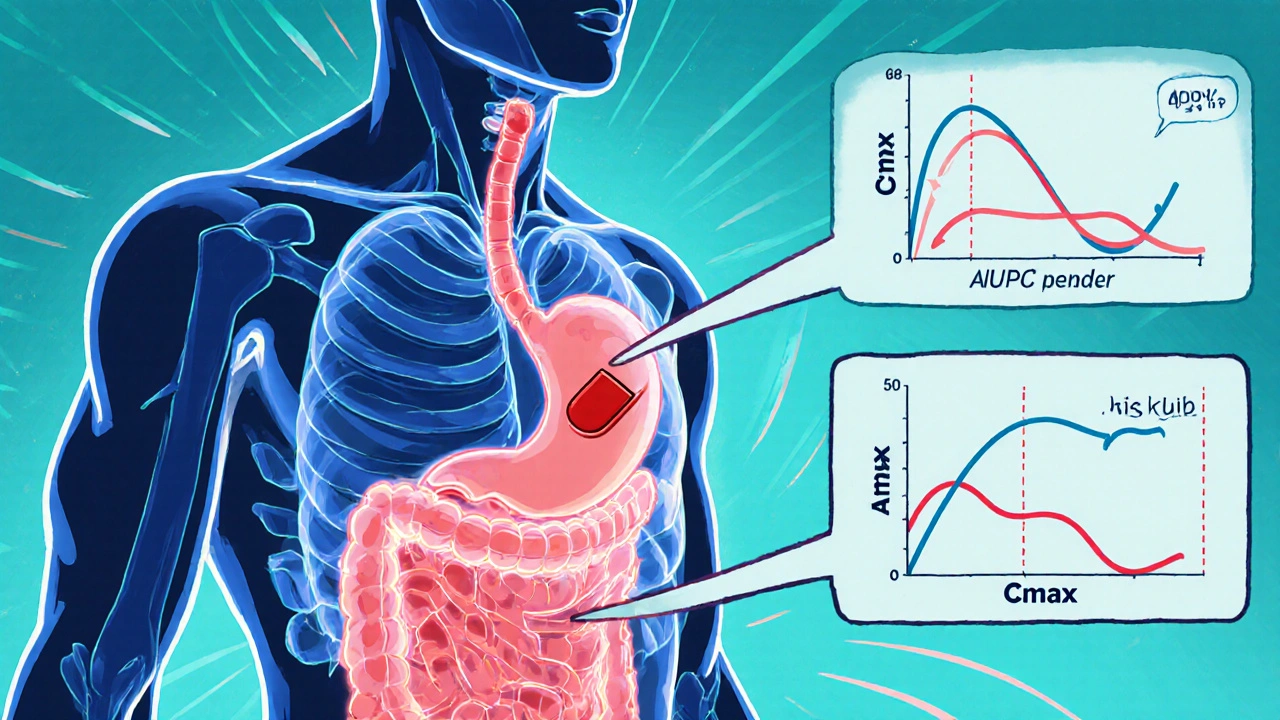
Bioequivalence Criteria: What Makes Generic Drugs Truly Equal
When you pick up a generic pill, you’re counting on it to do the same job as the brand-name version. That’s where bioequivalence criteria, the scientific standards used to prove a generic drug performs the same way in the body as its brand-name counterpart. Also known as therapeutic equivalence, it’s the backbone of affordable medicine. The FDA doesn’t just accept claims—it demands proof. Generic manufacturers must show their drug releases the same amount of active ingredient at the same rate as the original. If the blood levels of the drug don’t match within strict limits, the generic gets rejected. This isn’t guesswork—it’s measured with real human trials, using blood samples taken over hours to track absorption and elimination.
But not all generics are created equal, even if they pass the official tests. biosimilars, complex copies of biologic drugs made from living cells, not simple chemicals face even tougher standards because tiny differences in manufacturing can change how they work. Meanwhile, older chemical generics—especially those made overseas—have raised red flags. Some clinicians report higher rates of side effects, and FDA inspections have found inconsistent quality in certain factories. That’s why a drug might pass bioequivalence criteria on paper but still cause unexpected reactions in real life. It’s not always about the active ingredient—it’s about the fillers, coatings, and how the tablet breaks down in your stomach.
FDA approval, the official green light given after rigorous testing of safety, strength, and consistency doesn’t mean every batch is perfect. Manufacturing conditions, raw material sources, and even storage can vary. That’s why some patients notice differences when switching between generic brands—even if both are labeled as the same drug. The bioequivalence criteria set the floor, not the ceiling. What you want is a generic that’s not just approved, but reliably consistent.
These aren’t just technical details—they affect your health. If you’re on thyroid medication, blood thinners, or epilepsy drugs, even small changes in absorption can throw off your treatment. That’s why some doctors stick with brand names, and why patients should speak up if they feel a generic isn’t working the same. The system works most of the time, but it’s not flawless. The posts below dig into real cases where bioequivalence failed, where cost-cutting led to quality issues, and how to tell if your generic is truly safe and effective.
Bioequivalence Studies: What the FDA Requires Generic Drug Manufacturers to Prove
- Robin Tudge
- November 29, 2025
- 8 Comments
Learn what the FDA requires generic drug makers to prove through bioequivalence studies to ensure their products work just like brand-name drugs. Key criteria, exceptions, and recent updates explained.
read more